Abstract
Introduction
Increasingly clinicians face the choice between CPX-351 (NCT01696084) and venetoclax and azacitadine (ven/aza, NCT02993523) in front-line acute myeloid leukemia (AML), especially for frailer older adults with comorbidities. Given no prospective data, we examined retrospective observational survival outcomes for patients with newly diagnosed AML receiving either CPX-351 or ven/aza as initial therapy.
Methods
After local Institutional Review Board approval, we included patients from both the University of Pennsylvania ("HUP", n=111) and the Flatiron Health electronic health record (EHR) derived, de-identified database with a first incident AML diagnosed between January 2017 and 2021, and with documented receipt of CPX-351 or venetoclax and azacitadine as initial therapy (n=548). Given a total of 219 patients received CPX-351 and 440 received ven/aza, there was 90% power to detect a hazard ratio (HR) of 0.77 or smaller with alpha 0.05. Overall Survival (OS) was analyzed by the Kaplan-Meier method and compared between arms using the log-rank test. Pre-planned sensitivity analyses included restriction and multiple imputation (MI) to address missingness with inverse-probability of treatment weighting (IPTW) to balance baseline covariates with treatment effect estimates from each imputed dataset combined via Rubin's rules.
Results
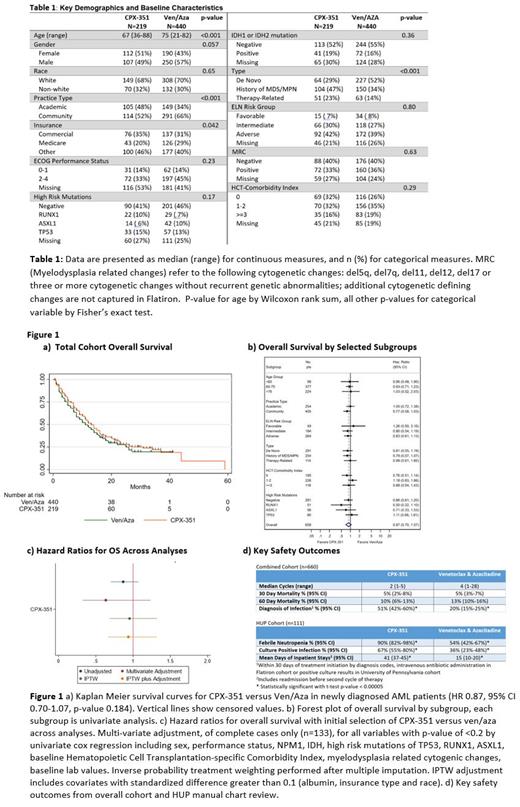
For all patients, baseline covariates showed patients receiving ven/aza were older (75 vs 65 years old median age), more likely to have Medicare (29% vs 20%), have de-novo AML vs secondary or therapy-related disease (52% vs 29%) and be treated in the community rather than an academic center (66% vs 52%). All other baseline covariates-including ELN cytogenetic risk groups, high risk mutations (TP53, ASXL1, RUNX1), FLT3, IDH, HCT-comorbidity index-were not statistically significant different between groups (table 1). Missing values in the HUP dataset was <5% for all baseline covariates; Flatiron dataset missing values ranged from 0-56% for covariates (e.g., sex and baseline LDH respectively).
Median OS (mOS) was 12 months for all patients and 13 months for CPX-351 versus 11 months for ven/aza (HR 0.87, 95% CI 0.70-1.07, p-value 0.184, Fig 1a). There was no clear subset with improved OS with CPX-351 vs ven/aza (Fig 1b); in multi-variate analysis controlling for all covariates with univariate p-value < 0.20 induction choice did not affect overall survival (limited to complete cases n=133, HR 0.63, p-value 0.73). Restricting to only the population eligible for CPX-351 pivotal trial, there was no significant difference in OS (CPX-351 n=160 mOS=10, ven/aza n=181 mOS=12 HR 0.86 p-value 0.33). After MI and IPTW, survival remained similar with HR 0.95 p-value 0.77 (Fig 1c). Adjusting further for variables with absolute standardized difference >10% after MI and IPTW (baseline HCT-comorbidity index, race and insurance status), multivariable Cox model gave similar results (HR 0.93, p-value 0.70).
Regarding safety outcomes, early mortality was similar. However, documented diagnosed infections were higher in CPX-351 patients and within the HUP cohort, rates of febrile neutropenia and culture positive infection were also higher (Fig 1d). Length of stay, including any admission prior to next cycle of therapy, was more than twice as long for CPX-351 in the HUP cohort.
Additional analyses, including detailed assessment of baseline covariates, multivariate analysis with transplant as a time-varying dependent covariate, event free survival, disease free survival also available for presentation.
Conclusion
In this multi-center real word dataset with over 600 patients, there was no statistically significant difference in overall survival between induction with CPX-351 and ven/aza. This large retrospective dataset is limited by missingness in covariates, risk of misclassification bias and lack of power for formal equivalence testing. However, a consistent lack of superiority for CPX-351 or ven/aza within a robust, manual chart-review of an academic institution, a large national real world database and combined analyses of these cohorts supports clinical equipoise. Given the apparent lack of a large survival benefit further work is needed. Prospective studies to confirm similar effectiveness with careful attention to side effects, quality of life and impact on transplant outcomes may help clinicians decide between these therapies.
Perl: Syndax: Consultancy; Loxo: Consultancy; Forma: Consultancy; AbbVie: Consultancy, Research Funding; Roche: Consultancy; Actinium: Consultancy; Genentech: Consultancy; Astellas: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Fujifilm: Research Funding; BMS/Celgene: Consultancy; Arog: Research Funding; Onconova: Consultancy; Sumitomo Dainippon: Consultancy. Luger: Syros: Honoraria; Agios: Honoraria; Daiichi Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria; Brystol Myers Squibb: Honoraria; Acceleron: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Onconova: Research Funding; Celgene: Research Funding; Biosight: Research Funding; Hoffman LaRoche: Research Funding; Kura: Research Funding. Frey: Kite Pharma: Consultancy; Novartis: Research Funding; Sana Biotechnology: Consultancy; Syndax Pharmaceuticals: Consultancy. Gill: Interius Biotherapeutics: Current holder of stock options in a privately-held company, Research Funding; Carisma Therapeutics: Current holder of stock options in a privately-held company, Research Funding; Novartis: Other: licensed intellectual property, Research Funding. Hexner: Blueprint medicines: Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees; Tmunity Therapeutics: Research Funding. Porter: American Society for Transplantation and Cellular Therapy: Honoraria; ASH: Membership on an entity's Board of Directors or advisory committees; DeCart: Membership on an entity's Board of Directors or advisory committees; Genentech: Current Employment, Current equity holder in publicly-traded company; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Unity: Patents & Royalties; Wiley and Sons Publishing: Honoraria. Stadtmauer: Oncopeptides: Consultancy; Amgen: Consultancy; BMS Celgene: Consultancy; GSK: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding. Maillard: Regeneron: Research Funding. Margolis: Pfizer: Consultancy; Leo: Consultancy; Sanofi: Consultancy; National Eczema Association: Membership on an entity's Board of Directors or advisory committees. Pratz: Abbvie: Consultancy, Honoraria, Research Funding; Agios: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Cellgene: Consultancy, Honoraria; Millenium: Research Funding; University of Pennsylvania: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal